Product Description
Ibandronate Sodium 99% Cas 138844-81-2 for Hypercalcemia and Anti-Osteoporotic biggest manufacturer Powder with top quality from China !

|
Batch No.
|
WG181222
|
Quantity
|
22.8kgs
|
|
Mfg. Date
|
Dec. 22, 2018
|
Package
|
As required
|
|
Rep. Date
|
Dec. 25, 2018
|
Exp. Date
|
Dec 21, 2021
|
|
Items
|
Standards
|
Results
|
|
Appearance
|
White to off-white crystalline powder
|
Complies
|
|
Identification
|
By IR
|
Complies
|
|
By HPLC
|
Complies
|
|
By Sodium salt reaction
|
Complies
|
|
Solubility
|
Freely soluble in water, slightly soluble in methanol
|
Complies
|
|
Clarity of Solution
|
Colorless, transparent
|
Complies
|
|
pH
|
4.0~4.8
|
4.3
|
|
Water Content
|
4.5%~6.5%
|
5.8%
|
|
Residue on Ignition
|
≤ 0.50%
|
Complies
|
|
Heavy Metals
|
≤ 10ppm
|
Complies
|
|
Phosphite
|
≤ 0.30%
|
0.10%
|
|
Phosphate
|
≤ 0.30%
|
0.05%
|
|
Sulphate
|
≤ 0.02%
|
Complies
|
|
Chloride
|
≤ 0.05%
|
Complies
|
|
Residual Solvent
|
Isopropanol: ≤5000ppm
|
Complies
|
|
Actone: ≤5000ppm
|
Complies
|
|
Related Substances
|
Maximum single impurity: ≤0.20%
|
0.09%
|
|
Total impurities: ≤1.0%
|
0.40%
|
|
Assay
|
99.0%~102.0%
|
99.92%
|
|
Reference Standard
|
In-house Standard
|
|
Conclusion
|
The product complied to In-house standard.
|
|
Storage
|
Preserve in tight,light-resistant containers in a cool place
|
Function :
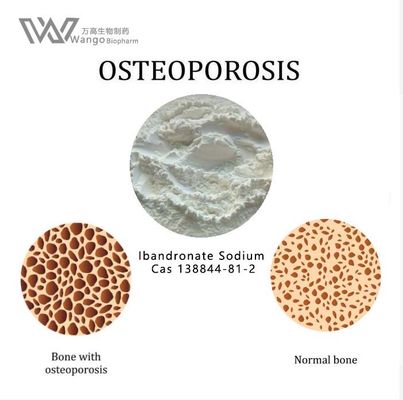
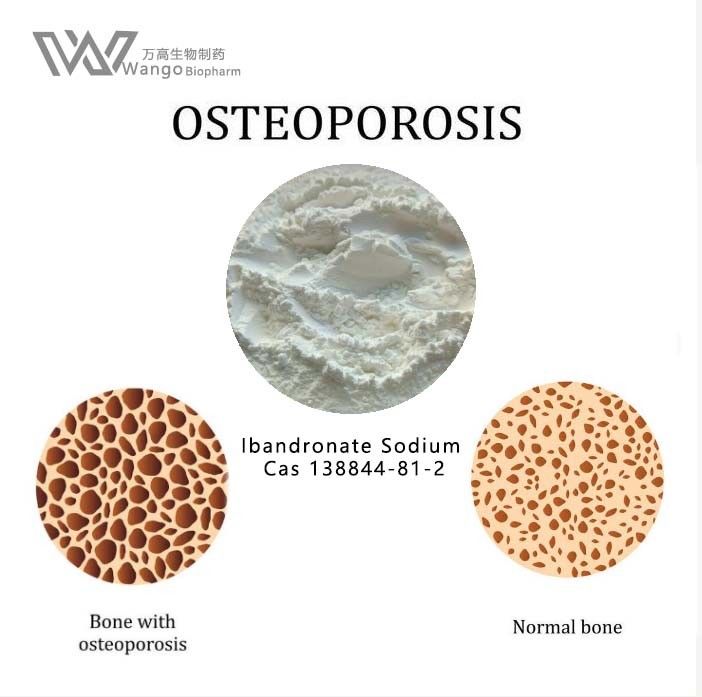
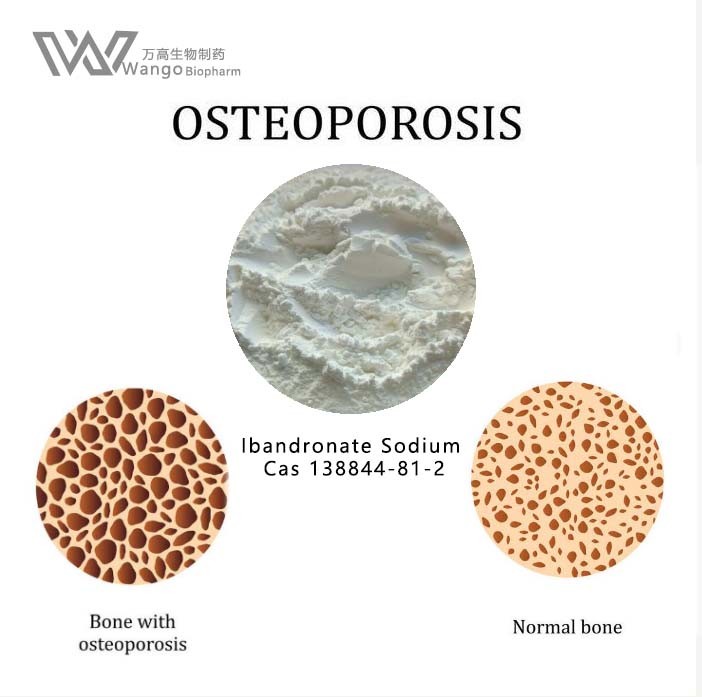
Ibandronate sodium is commonly used to treat osteoporosis, mainly for postmenopausal osteoporosis caused by excessive calcium loss. Secondly, iban sodium phosphate can also treat malignant tumor diseases, such as bone tumor, or bone metastasis of other tumor diseases. These diseases will lead to the symptoms of bone pain in patients, so they can take iban Sodium for conditioning and relief of symptoms. Some patients will have fever, muscle pain and other symptoms after taking ibandronate sodium, so it is necessary to strictly follow the doctor's guidance to take it.
Pharmacokinetics
Its mechanism of action is identical to the other bisphosphonate agents. Administered daily (2.5 mg), ibandronate has been clinically shown to reduce the risk of vertebral fractures by 62%. If administered on an intermittent basis (20 mg), it reduces the risk of vertebral fractures by 50%. Ibandronate (2.5 mg daily), along with 500 mg of supplemental calcium, has been clinically shown to increase BMD in the hip (1.8%), femoral neck (2.0%), and lumbar spine (3.1%). The 150-mg formulation approved in March 2005 represents the first oral therapy for a chronic disease to be administered once monthly.
Clinical Use
Ibandronate sodium was approved in May 2003 for the treatment and prevention of osteoporosis in postmenopausal women.
Side effects
Adverse events as sociated with the injectable form ulation included arthralgia, back and abdominal pain, and hypertens ion. There is a risk of renal toxicity that is inversely related to the rate of administration of this formulation.
Metabolism
The oral bioavailability of this agent is extremely poor (0.6%) and is adversely affected by the presence of food, beverages other than water, and other medications, including calcium or vitamin D supplements and antacids. Because of the increased calcium content in mineral water, patients should not take this medication with this type of water. Drugs that inhibit gastric acid secretion (e.g., H2 antagonists and proton-pump inhibitors) actually promote ibandronate absorption. Like the others in this therapeutic class, ibandronate is not metabolized, and that which is not bound to the bone (40–50% of the absorbed dose) is eliminated renally unchanged. It does not inhibit the cytochrome P450 (CYP450) isozymes. This agent does not require any dosage adjustment for patients with hepatic impairment or mild to moderate renal impairment (creatinine clearance, >30 mL/min). Ibandronate should not be prescribed for patients with severe renal impairment (creatinine clearance, <30 mL/min)
FAQ
Q1: Are you a manufacturer?
Yes, Wango Biopharm have concentrated on Organic synthesis over 12 years since 2008.
Advanced R&D technology, 380,000 categories chemical reagent are in stock.
Trading products we will serve for you with our ISO supplier system, making your purchasing risk into the lowest.
In addition, we provide the customization service of a new compound from lab to bulk manufacturing.
Q2: How to contact with us?
You can contact with us on Made-in-China, the Average Response Time is 0-4h on Work time, <24h on Non-work time.
For every inquiry, our sales will provide the one-for-one service for you.
Q3:Which kind of payment do you accept?
Normal orders: Company account(The price will be 5%-13% extra discount)
Sample/small orders: Paypal(The 5-10% extra fee),Western Union etc.
About the payment term, we will negotiate alone.
Q4:How to confirm the Product Quality before placing orders?
1st, you can get free samples for some products, just pay the basic cost for us.
2nd,Also you can send the specification or your quest to us, we will customize the products for you.
3rd,We emphasis that our whole company make a living with organic chemical and customers, we absolutely impossible to sale any fake goods, unqualified goods, it is shame! And None False certificate/lie/illegal thing in our sales net!
Q5:How do you treat quality complaint?
The customer complaint handling procedures is the key part in Our ISO management system. We will set up an investigation team for your complaint, and give the handling plan and our corrective and preventive measures within a limited time.
Q6:Package
We will refer the MSDS to design the package standard and show you at the PI or Quotation list.
If you want customize package and haven't go against the principle of science, we will do as your request. The cost will be calculate your request.
For dangerous goods, only one style of package, we will do as the UN standard.
Q7:Shipping
We have R&D and manufactured chemicals for 12 years, we have rich experience on Shipping, Many famous companies cooperated with us stably, we are good at shipping all kinds of chemicals.
General goods: The best service, price and lead time.
Dangerous goods: The professional cooperated company, the details please check our website.


 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!