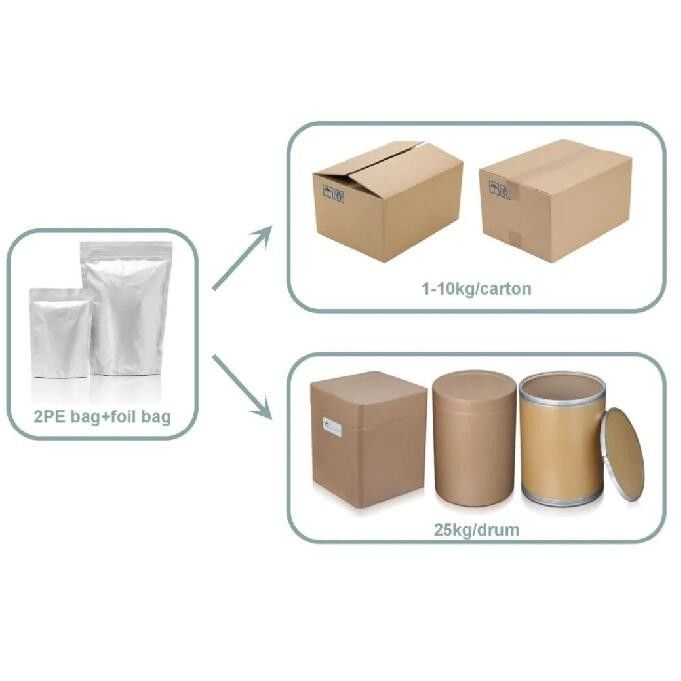
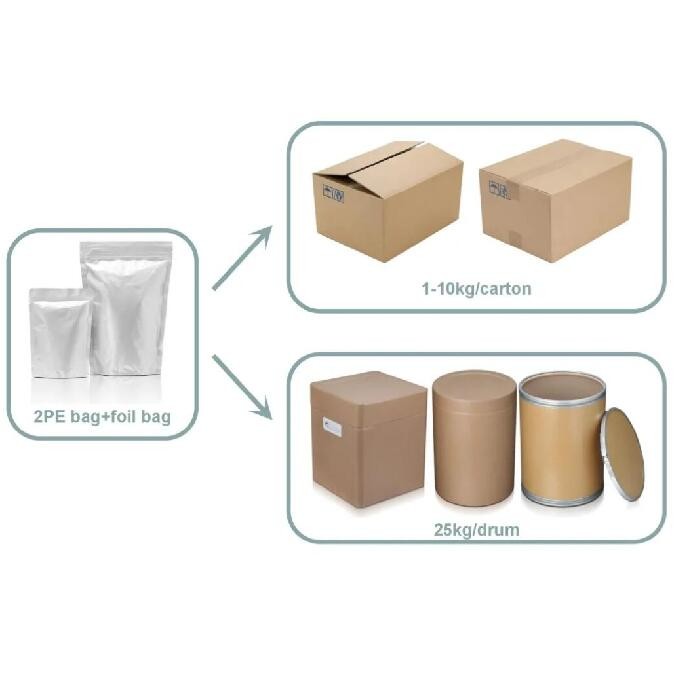
Product Description
Citicoline Sodium (CDPC) 99% enhance the function of brain stem reticular formation Medical Raw Material Powder Cdp-Choline Fast Delivery CAS: 33818-15-4
| Item |
Specification |
Result |
| Description |
white crystalline powder,odorless |
Conforms |
| Solubility |
Easily soluble in water, unsoluble in ethanol |
Conforms |
| PH |
6.0~7.5 |
6.9 |
| Solution clarity and color |
solution should be clear and colorless |
Conforms |
| Related substances |
5’CMP 0.3%
Simple impurity
Total other impurity
|
0.01%
0.03%
0.10%
|
| Chloride |
0.05% |
Conforms |
| Ammonium |
0.05% |
Conforms |
| Fe |
0.01% |
Conforms |
| Phosphate |
0.1% |
Conforms |
| Loss on drying |
≤6.0% |
1.3% |
| Heavy metals |
0.002% |
Conforms |
| Arsenide |
0.0001% |
Conforms |
| Bacterial Endotoxins |
0.3EU/mg |
Conforms |
| Microbial Limit |
Conforms |
Conforms |
| Assay(on dried basis) |
≥99.0% |
99.2% |
| Conclusion: The results conform with CP2010 |

What is DCPC , Cytidine 5′-diphosphocholine, Cytidine diphosphate-choline ?
also known as cytidine diphosphate-choline (CDP-Choline) & cytidine 5'-phosphocholine is a psychostimulant/
nootropic. It is an intermediate in the generation of phosphatidylcholine from choline. Studies suggest that CDP-choline supplements increase dopamine receptor densities, and suggest that CDP choline supplementation helps prevent memory impairment resulting from poor environmental conditions. Preliminary research has found that citicoline supplements help improve focus and mental energy and may possibly be useful in the treatment of attention deficit disorder. Citicoline has also been shown to elevate ACTH independently from CRH levels and to amplify the release of other HPA axis hormones such as LH, FSH, GH, and TSH in response to hypothalamic releasing factors. These effects on HPA hormone levels may be beneficial for some individuals but may have undesirable effects in those with medical conditions featuring ACTH or cortisol hypersecretion including, but not limited to, PCOS, type II diabetes, and major depressive disorder.
Clinical Overview
Use
There is mounting evidence for choline's place in therapy for stroke, brain and spinal cord injury, cognitive deficits, and glaucoma; however, results in clinical trials have been inconsistent.
Oral dosages of 250 to 2,000 mg daily have been evaluated in adolescents and adults in clinical trials. Lower doses (100 mg twice daily) have been used in short-term trials (6 weeks) with combination therapy in patients with major depressive disorder.
Contraindications
Contraindications have not been identified.
Pregnancy/Lactation
Information regarding safety and efficacy in pregnancy and lactation is lacking at dosages above those usually taken nutritionally.
Interactions
None well documented.
Adverse Reactions
Citicoline was well tolerated in clinical trials. Adverse effects may include GI disturbances, transient headaches, hypotension, tachycardia, bradycardia, and restlessness.
Toxicology
Studies in humans are limited.
Citicoline is found in all animal and plant cell membranes. It is available commercially in its free-base form or as a sodium salt.
History
Citicoline is widely available internationally as a supplement, which was originally developed in Japan for the treatment of cerebrovascular disorders. Use of citicoline has been extended to include treatment of chronic conditions, although further research is needed.
Chemistry
Citicoline is a phospholipid composed of ribose, pyrophosphate, cytosine, and choline. It is water-soluble and highly bioavailable.3 Citicoline is produced endogenously as an intermediate in the production of phosphatidylcholine from choline and is then hydrolyzed in the small intestine to make choline and cytidine available for further biosynthesis.
Supplementation with citicoline increases choline stores available for other biosynthetic pathways. Citicoline appears to decrease glutamate levels in the brain and increase adenosine triphosphate, which in turn offers protection against ischemic neurotoxicity. Increased glucose metabolism in the brain and cerebral blood flow has also been demonstrated, as well as increased availability
,
Cocane dependence
Clinical data
Studies have investigated a role for citicoline in substance addiction and among patients with bipolar disorder. A 12-week, double-blind, parallel-group, randomized, placebo-controlled trial (n = 130) in adults with bipolar disorder and cocane dependence reported a significant early treatment effect in favor of citicoline (500 mg/day titrated up every 2 weeks to 2,000 mg/day by week 6) compared to placebo.
Cognition
Animal data
Accelerated resynthesis of phospholipids and subsequent protection of cell membranes in the presence of citicoline have been suggested as a possible mechanism of action, based on animal studies. Labeled phospholipid from radioactively labeled citicoline has been shown to cross the blood-brain barrier. Studies in rats with cognitive impairment have been conducted, and improved memory and learning have been demonstrated in older rats and those with induced memory deficits. Citicoline has also demonstrated enhanced learning ability in dogs. Limited animal studies suggest that citicoline may counteract the deposition of beta-amyloid involved in Alzheimer disease.
Clinical data
A Cochrane meta-analysis of clinical trials up to 2004 found some evidence of supplemental citicoline's positive effect on memory and behavior in the short- to medium-term versus placebo. Effect size for memory measures (N = 884) was 0.19 (95% confidence interval [CI], 0.06 to 0.32), and for the measure of positive Global Clinical Impression (N = 217), an odds ratio (OR) of 8.89 was found (95% CI, 5.19 to 15.22). The report further suggests that the effect of citicoline (oral or intravenous) on memory in the included studies did not appear to depend on the pathogenesis of the cerebral disorder. Trials included in the meta-analysis enrolled participants with mild to moderate dementia and Alzheimer disease, as well as those with cerebrovascular disorders.An open-label IDEALE study in Italy administered citicoline 1 g daily in 2 divided doses over 9 months to 265 patients with mild age-related vascular cognitive impairment. Mini-Mental State Examination scores remained essentially unchanged over time for the treatment arm, while a decline was evident in the control patients.Another open-label, parallel study of citicoline versus usual treatment was conducted in 347 poststroke patients in Spain. Improved cognitive outcomes were reported for the citicoline-treated group in attention, temporal orientation, and functional outcome measures.
Depression
Clinical data
Depression scores improved significantly at 2, 4, and 6 weeks from baseline in patients diagnosed with major depressive disorder who received 6 weeks of citicoline (100 mg every 12 hours) in combination with citalopram (20 mg/day × 7 days, then 40 mg/day) compared to citalopram alone (P<0.03, P=0.032, and P=0.021, respectively) in a randomized, double-blind, placebo-controlled, parallel-group study (n=50). Additionally, the "depressed mood" item improved significantly at the end of the trial (P=0.04). Remission rate was also significantly greater with citicoline combination therapy (72%) compared to citalopram only (44%; P=0.045). No significant differences were noted in adverse events between groups.
Glaucoma
Animal data
Studies in animals suggest that citicoline stimulates dopamine in the retina.
Clinical data
Limited trials have been conducted. However, an 8-year follow-up of patients with glaucoma included in an earlier trial showed improvement in retinal and visual function. An open-label study showed similar effects following 2 weeks of treatment with oral citicoline 1 g daily.
Head injury
Animal data Antioxidant and anti-inflammatory mechanisms of citicoline have been evaluated in experimental studies in rats.
Clinical data
A 2008 systematic review of the effect of cholinomimetic agents on head injury included trials using citicoline and case reports, all with some limitations in the methodology (eg, small sample size, single blinding). Positive findings have been reported in these studies; however, in the larger Citicoline Brain Injury Treatment Trial published in 2012, a 90-day regimen of enteral or oral citicoline 2,000 mg daily did not result in improvement in functional and cognitive status versus placebo (global OR, 0.87 [95% CI, 0.72 to 1.04]).
Psychomotor function
Clinical data
Attention and psychomotor speed (of the dominant hand) improved significantly in healthy adolescent males (13 to 18 years of age) after 28 days of supplementation with citicoline 250 and 500 mg/day compared with placebo in a randomized, double-blind trial (n = 75). Changes in scores were predicted significantly more by a weight-adjusted dose with greater improvements in accuracy, detectability, and commission errors following higher weight-adjusted doses.
FAQ
Q1: Can i get some samples ?
A: Yes, we can supply the free sample, but the shipping cost be paid by our customers.
Q2: How to start orders or make payments
A: Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T, Western Union or Paypal or Escrow(Alibaba).
Q3: How to confirm the Product Quality before placing orders ?
A:You can get free samples for some products,you only need to pay the shipping cost or arrange a courier to us and take the samples. You can send us your product specifications and requests,we will manufacture the products according to your requests.
Q4:What's your MOQ ?
A:Our MOQ is 1kg. But usually we accept less quantity such as 100g on the condition that sample charge is 100% paid.
Q5: How about delivery leadtime ?
A:Delivery lead time: About 3-5 days after payment confirmed. (Chinese holiday not included)
Q6:Is there a discount ?
A:Different quantity has different discount.
Q7: How do you treat quality complaint ?
A:First of all, our quality control will reduce the quality problem to near zero. If there is a real quality problem caused by us, we will send you free goods for replacement or refund your loss.


 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!